Click here for downloadable PDF


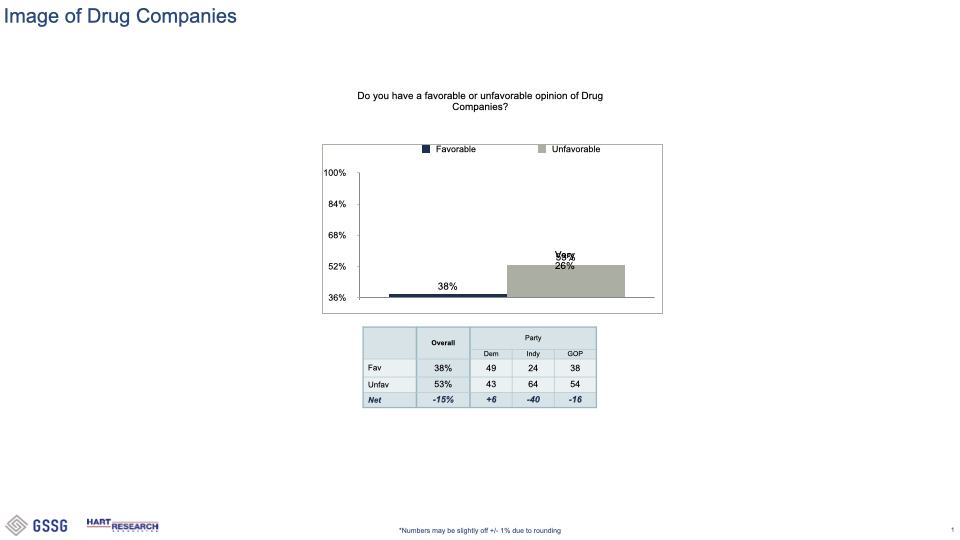
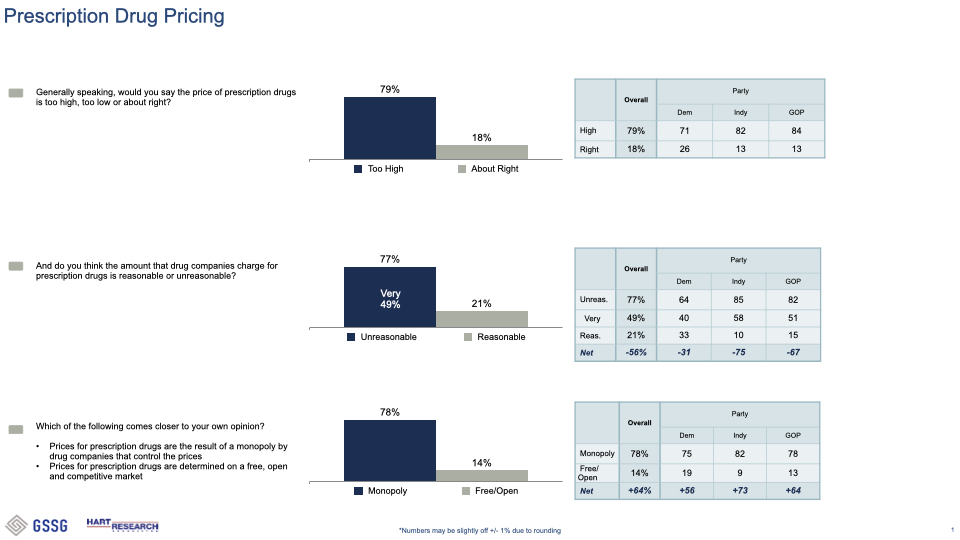
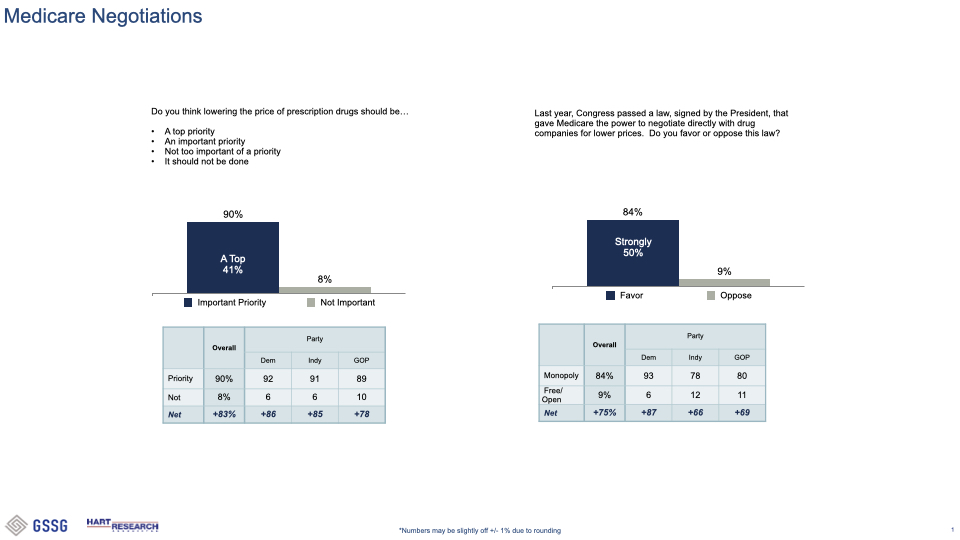
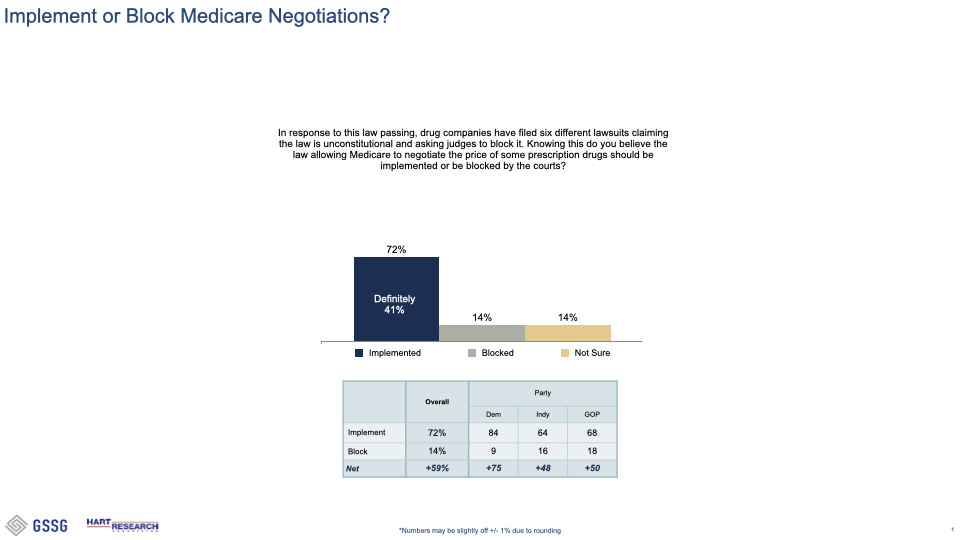
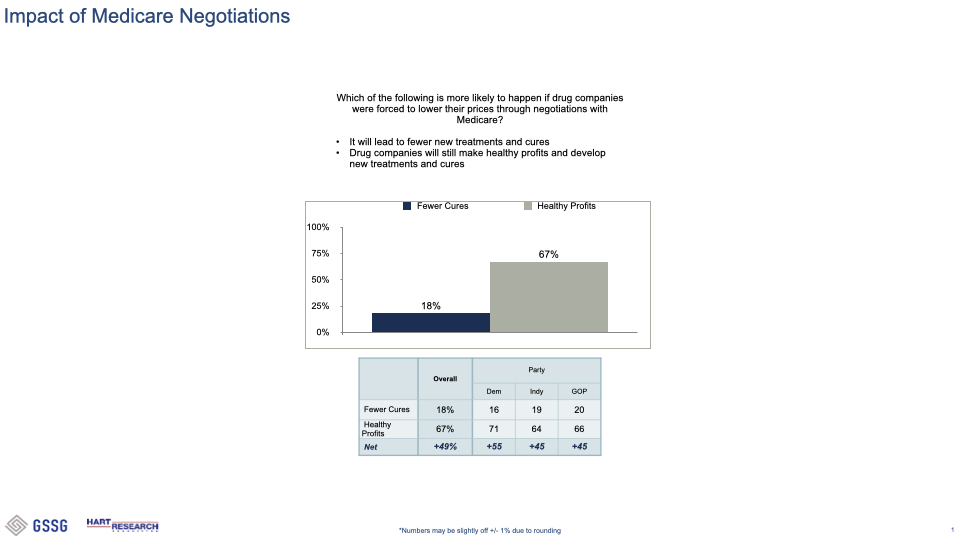
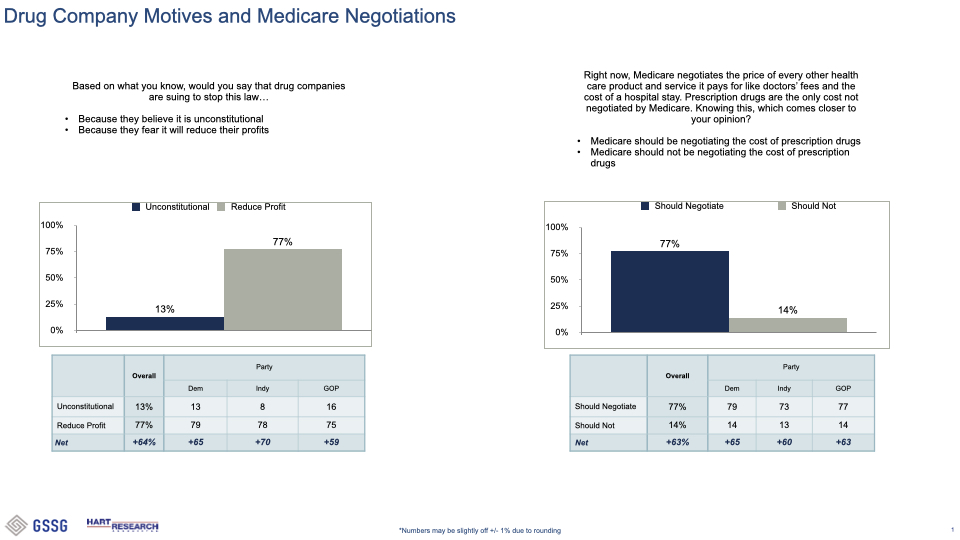
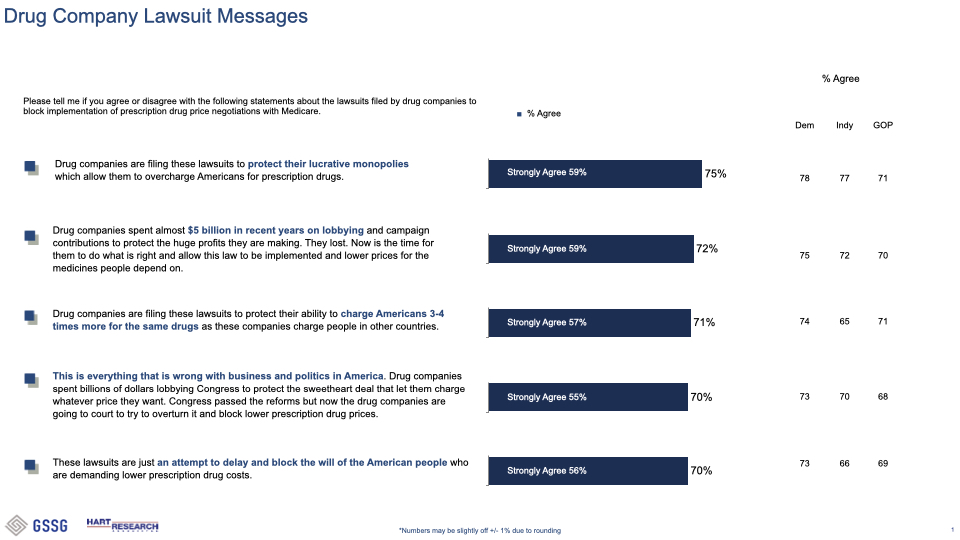
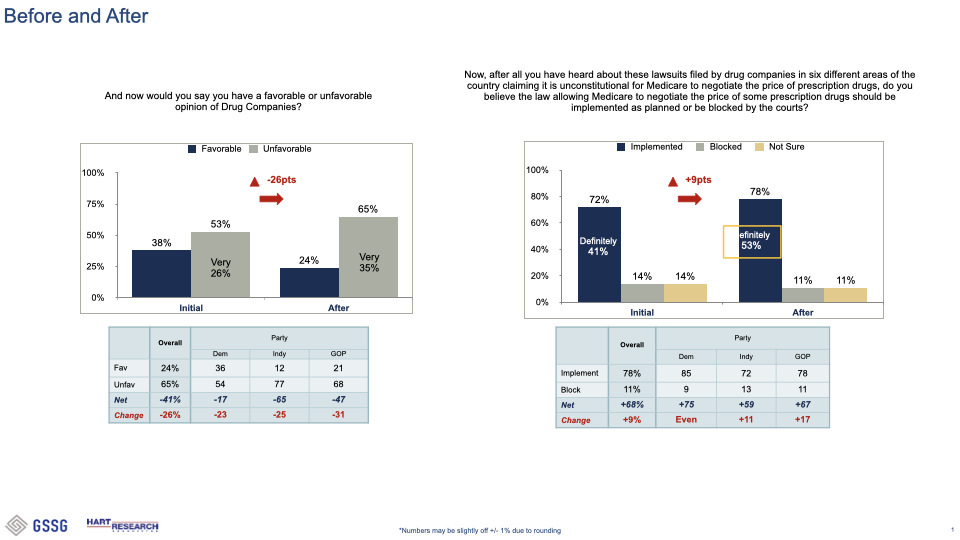
###
The Bills Aim To Curb Patent Abuses By Drug Companies, And Shine A Light On Secret Practices Of Pharmacy Benefit Managers
WASHINGTON, D.C. — The following statement was issued by Merith Basey, executive director of Patients For Affordable Drugs Now, in response to the Senate Judiciary Committee’s vote to advance a package of five bipartisan bills that would lower drug prices and promote both competition and innovation by curbing anticompetitive behavior committed by pharmaceutical corporations and pharmacy benefit managers (PBMs):
“On behalf of patients all across the country, thank you Chairman Durbin, Ranking Member Graham, and members of the Senate Judiciary Committee for passing legislation to help fix key elements of our rigged drug price system. We fully support the bills to crack down on patent abuse, increase coordination between the FDA and USPTO to ensure patents are used to reward innovation and not to unfairly block competition, as well as investigate the practices of pharmacy benefit managers (PBMs). Together these bills can help to restore balance to our drug price system and increase competition to lower drug prices for patients.
“We urge Senate Majority Leader Schumer to bring these bills to the floor for a vote as soon as possible, and we call on each senator to support the passage of this bipartisan package intact without any weakening amendments from Big Pharma.”
###
Background:
January 2023: Millions Of Patients Will Pay Less For Medication Thanks To Inflation Reduction Act
This Month, Insulin Copays Are Capped At $35 And All Adult Vaccines Are Free Under Medicare Part D
WASHINGTON, D.C. — January 1, 2023, marked a milestone for drug price reforms in the United States. Thanks to the Inflation Reduction Act, for the first time ever, all patients who receive their insulin through Medicare Part D (including most forms of insulin delivered via syringes and pens) now have their copays capped to $35 per month, and all beneficiaries now face $0 out of pocket for all vaccines covered by the drug program.
“2023 marks a momentous year for patients – millions of people in the U.S. will begin to feel the impacts of the historic drug price reforms in the Inflation Reduction Act, both on their health and well being as well as in their wallets,” said Merith Basey, executive director of Patients For Affordable Drugs Now. “While we’re delighted to begin the year knowing that millions of people on Medicare Part D will now have their insulin copays limited to $35 a month and will have access to free vaccines, we acknowledge that there is so much more to be done. This is just the beginning.”
The insulin copay cap includes most forms of insulin delivered via syringes and pens (insulin delivered via pumps will be capped in July 2023). About 2.7 million Medicare beneficiaries will experience savings from the January insulin copay cap, and savings are projected to average around $850 a year per beneficiary.
“I live with high blood pressure as well as insulin-dependent diabetes,” said Patricia McKenzie, a Medicare beneficiary who lives in Lithonia, GA, and receives her Humalog insulin through Part D. “I live on a fixed income, so I have to plan carefully in order to afford my prescriptions. The new $35 copay cap for my insulin will ensure I can afford my insulin for as long as I need it.”
Steven Hadfield lives with a rare blood cancer as well as type 2 diabetes and takes Lantus insulin. “My Lantus insulin carries a monthly list price of $283, which only adds to the large financial burden of my other drugs,” Steven of Charlotte, NC, who gets his insulin through the Part D drug program, shared. “Over the past year, I’ve gone without my Lantus at times because of its cost. Now, it will only cost me $35 which will bring me more consistency and, for the first time, lower my drug costs.”
For the millions of Medicare beneficiaries who receive vaccines each year, access to free vaccines will bring relief. Expensive vaccines such as Shingrix, which treats shingles, had cost about $200 out of pocket for Medicare beneficiaries, but are now free. And Medicare beneficiaries will continue to receive COVID-19 vaccines and boosters for free.
“When I got my two shingles vaccines, they cost over $200 out of pocket, even with Medicare,” said patient advocate Jackie Trapp of Muskego, WI, who lives with multiple myeloma, an incurable blood cancer. “Now that vaccines are free for Medicare beneficiaries, I’m so relieved that future patients like me won’t have to spend what I did just to protect themselves from diseases like shingles.”
All of the drug price reforms in the Inflation Reduction Act will continue to be implemented in the coming months and years. In addition to capping insulin copays and making vaccines free, drug companies will finally be penalized for raising prices above the rate of inflation in 2023. Over the next three years, Medicare will also begin to negotiate lower drug prices directly with drug companies; and starting in 2025, Medicare Part D beneficiaries will have their out-of-pocket prescription costs capped starting at $2,000 a year.
For more information on the implementation of the drug price reforms in the Inflation Reduction Act, visit medicarenegotiation.org.
###
P4ADNow Urges Congress To Prioritize Patients By Promoting Competition And Lowering Drug Prices By Including Bipartisan Reform To FDA’s Citizen Petition Process In End-Of-Year Budget Package
WASHINGTON, D.C. — As Congress works towards finalizing an end-of-year budget package, Patients For Affordable Drugs Now (P4ADNow) sent a letter today urging the Senate Committee on Health, Education, Labor and Pensions and the U.S. House Committee on Energy and Commerce to boost generic competition and lower prescription drug prices by including the bipartisan legislation Ensuring Timely Access to Generics Act (S.562) in the end-of-year budget package. This bill addresses abuse of the Food and Drug Administration’s (FDA) citizen petition process in order to speed approval of more affordable generic drugs to lower prices for patients and save the government hundreds of millions of dollars. P4ADNow was joined on the letter by the Alliance of Community Health Plans (ACHP), American College of Physicians, American Society of Health-System Pharmacists (ASHP), Blue Cross Blue Shield Association, The Campaign for Sustainable Rx Pricing, Friends of Cancer Research, and Protect Our Care.
“In order to restore the intended purpose of the citizen petition process and ensure timely market entry of competition, we urge you to include the bipartisan Ensuring Timely Access to Generics Act in the year-end fiscal package,” reads the letter. “CBO reports the reform will save the government $207 million over ten years.”
Currently, the citizen petition process is often misused by brand name drug manufacturers that submit sham petitions in an effort to delay or block generic approval and market entry. The Ensuring Timely Access to Generics Act (S.562), sponsored by Sens. Shaheen (D-NH), Cassidy (R-LA), Bennet (D-CO), and Rubio (R-FL), would strengthen the citizen petition process, more effectively weeding out petitions that are filed with the intent to delay the approval of generics or biosimilars. The bill restores the integrity of the process, decreases administrative burden on the FDA, and will improve speedy patient access to more affordable generics and biosimilars.
“Passing the bipartisan citizen petition bill would be a win-win for Congress – it boosts competition by decreasing barriers for cheaper generic drugs to come to market, driving down prices for patients and saving the government hundreds of millions of dollars,” said David Mitchell, a patient with incurable blood cancer whose drugs carry a list price of more than $900,000 per year and founder of Patients For Affordable Drugs Now. “We are grateful for Sens. Shaheen, Cassidy, Bennet, Rubio, and Baldwin’s leadership in advocating for this important reform to ensure the system works as intended for patients and consumers.”
In addition to the citizen petition bill, P4ADNow also supports inclusion of two other priority bills in Congress’ end-of-year budget – Retaining Access and Restoring Exclusivity (RARE) Act (S. 4185) and Increasing Transparency in Generic Drug Applications Act (H.R. 7032) – which will clarify orphan drug exclusivity to crack down on prolonged monopolies and enhance the FDA’s authority to communicate with generic competitors.
Read the full letter and list of signers here and below.
December 5, 2022
The Honorable Patty Murray
Chairwoman
U.S. Senate Committee on
Health, Education, Labor and Pensions
428 Senate Dirksen Office Building
Washington, DC 20510
The Honorable Richard Burr
Ranking Member
U.S. Senate Committee on
Health, Education, Labor and Pensions
428 Senate Dirksen Office Building
Washington, DC 20510
The Honorable Frank Pallone
Chairman
U.S. House Committee On Energy and
Commerce
2152 Rayburn House Office Building
Washington, DC 20515
The Honorable Cathy McMorris Rodgers
Ranking Member
U.S. House Committee on Energy and
Commerce
2152 Rayburn House Office Building
Washington, DC 20515
Dear Chairwoman Murray, Ranking Member Burr, Chairman Pallone, and Ranking Member McMorris Rodgers,
As Congress works toward finalizing an end-of-year budget package, we urge the chambers to include bipartisan legislation to address abuse of the Food and Drug Administration’s (FDA) citizen petition process in order to reduce drug prices and save the government hundreds of millions of dollars by speeding generics to market to increase competition. The Ensuring Timely Access to Generics Act (S.562), sponsored by Sens. Shaheen (D-NH), Cassidy (R-LA), Bennet (D-CO), and Rubio (R-FL), strengthens the FDA’s ability to reject citizen petitions if it believes the primary purpose of the petition is to delay approval of a generic competitor. CBO reports the reform will save the government $207 million over ten years. Sen. Baldwin (D-WI) offered the legislation as an amendment during the Senate HELP Committee’s mark-up of the FDA user fee reauthorization legislation; it passed with a strong, bipartisan 16-6 vote.
The citizen petition process at the FDA is intended to provide a forum for patients, consumer groups, and other entities to raise safety concerns about FDA decision making, including drug approvals. Too often, however, this process is co-opted by brand-name drug companies to delay competition. Currently, the FDA must delay authorization of any drug in order to process all citizen petitions. Brand drug companies often game the system by raising invalid, outdated, or extraneous issues in order to block or slow competition from generic products that are poised to enter the market. To that point, research has revealed that brand-name drug makers were behind 92% of all 505(q) citizen petitions filed between 2011 and 2015. Overwhelmingly, these petitions were “shams,” not raising legitimate safety concerns, which is why the FDA threw out nine of every 10 of these petitions.
Both the Trump and Biden administrations have identified submission of sham citizen petitions as a threat to timely approval of generics and competition. In 2018, Former FDA Commissioner Scott Gottlieb noted that manipulation of the process “can add to resource burdens on the generic drug review process and the FDA’s regulatory decision making” and decrease the speed of the approval process. In its 2021 drug pricing competition plan, the Biden administration also said that legislative changes were needed to “make it harder for brand manufacturers to abuse the regulatory process to prevent the introduction of biosimilar and generic products” such as through manipulation of the citizen petition process.
In order to restore the intended purpose of the citizen petition process and ensure timely market entry of competition, we urge you to include the bipartisan Ensuring Timely Access to Generics Act in the year-end fiscal package.
Sincerely,
Patients For Affordable Drugs Now
Alliance of Community Health Plans
American College of Physicians
American Society of Health-System Pharmacists
Blue Cross Blue Shield Association
The Campaign for Sustainable Rx Pricing
Friends of Cancer Research
Protect Our Care
CC: Leader Schumer, Speaker Pelosi, Minority Leader McConnell, and Minority Leader McCarthy
Click here for PDF of letter
December 5, 2022
The Honorable Patty Murray
Chairwoman
U.S. Senate Committee on
Health, Education, Labor and Pensions
428 Senate Dirksen Office Building
Washington, DC 20510
The Honorable Richard Burr
Ranking Member
U.S. Senate Committee on
Health, Education, Labor and Pensions
428 Senate Dirksen Office Building
Washington, DC 20510
The Honorable Frank Pallone
Chairman
U.S. House Committee On Energy and
Commerce
2152 Rayburn House Office Building
Washington, DC 20515
The Honorable Cathy McMorris Rodgers
Ranking Member
U.S. House Committee on Energy and
Commerce
2152 Rayburn House Office Building
Washington, DC 20515
Dear Chairwoman Murray, Ranking Member Burr, Chairman Pallone, and Ranking Member McMorris Rodgers,
As Congress works toward finalizing an end-of-year budget package, we urge the chambers to include bipartisan legislation to address abuse of the Food and Drug Administration’s (FDA) citizen petition process in order to reduce drug prices and save the government hundreds of millions of dollars by speeding generics to market to increase competition. The Ensuring Timely Access to Generics Act (S.562), sponsored by Sens. Shaheen (D-NH), Cassidy (R-LA), Bennet (D-CO), and Rubio (R-FL), strengthens the FDA’s ability to reject citizen petitions if it believes the primary purpose of the petition is to delay approval of a generic competitor. CBO reports the reform will save the government $207 million over ten years. Sen. Baldwin (D-WI) offered the legislation as an amendment during the Senate HELP Committee’s mark-up of the FDA user fee reauthorization legislation; it passed with a strong, bipartisan 16-6 vote.
The citizen petition process at the FDA is intended to provide a forum for patients, consumer groups, and other entities to raise safety concerns about FDA decision making, including drug approvals. Too often, however, this process is co-opted by brand-name drug companies to delay competition. Currently, the FDA must delay authorization of any drug in order to process all citizen petitions. Brand drug companies often game the system by raising invalid, outdated, or extraneous issues in order to block or slow competition from generic products that are poised to enter the market. To that point, research has revealed that brand-name drug makers were behind 92% of all 505(q) citizen petitions filed between 2011 and 2015. Overwhelmingly, these petitions were “shams,” not raising legitimate safety concerns, which is why the FDA threw out nine of every 10 of these petitions.
Both the Trump and Biden administrations have identified submission of sham citizen petitions as a threat to timely approval of generics and competition. In 2018, Former FDA Commissioner Scott Gottlieb noted that manipulation of the process “can add to resource burdens on the generic drug review process and the FDA’s regulatory decision making” and decrease the speed of the approval process. In its 2021 drug pricing competition plan, the Biden administration also said that legislative changes were needed to “make it harder for brand manufacturers to abuse the regulatory process to prevent the introduction of biosimilar and generic products” such as through manipulation of the citizen petition process.
In order to restore the intended purpose of the citizen petition process and ensure timely market entry of competition, we urge you to include the bipartisan Ensuring Timely Access to Generics Act in the year-end fiscal package.
Sincerely,
Patients For Affordable Drugs Now
Alliance of Community Health Plans
American College of Physicians
American Society of Health-System Pharmacists
Blue Cross Blue Shield Association
The Campaign for Sustainable Rx Pricing
Friends of Cancer Research
Protect Our Care
CC: Leader Schumer, Speaker Pelosi, Minority Leader McConnell, and Minority Leader McCarthy
Click here for PDF of letter
Groups Representing Patients, Consumers, Seniors, Unions, Small Businesses, Large Employers, And Physicians And Disease Advocacy Groups Align On Rx Reforms In BBB Act
| WASHINGTON, D.C. — As the Senate considers the terms of a reconfigured reconciliation package, 91 organizations representing patients, consumers, seniors, unions, small businesses, large employers, and physicians and disease advocacy groups sent a letter to all 50 Senate Democrats urging them to take immediate action to advance a reconciliation package that includes the reforms to lower prescription drug prices in the Build Back Better Act. “These drug pricing reforms are not controversial for the people of America; they are the most popular element of BBB. Over 80 percent of Americans support them — Democrats, Republicans, and independents alike,” said David Mitchell, a patient with incurable blood cancer whose drugs carry a list price of more than $900,000 per year and founder of Patients For Affordable Drugs Now. “The provisions before the Senate will help restore balance to ensure patients get the innovation they need at prices they can afford. All 50 Senate Democrats support the legislation; this is Congress’ chance to deliver on years of promises. This opportunity won’t come again soon.” “Millions of AARP members have told us they are sick of Big Pharma ripping them off by charging three times more for medicines in the U.S.,” said Nancy A. LeaMond, Executive Vice President and Chief Advocacy and Engagement Officer at AARP. “Congress has an historic opportunity to finally bring down the price of prescription drugs and help address the impacts of inflation. Voters are watching, and they are tired of waiting for Congress to act.” Just last week, 40 House Democrats sent a letter to congressional leadership calling on their colleagues to lower drug prices by passing the drug pricing reforms already agreed to in the Build Back Better Act. The drug price provisions under consideration will, for the first time, authorize Medicare to negotiate prices directly for some of the most expensive prescription medicines, including insulin; institute a hard cap on out-of-pocket drug costs for Medicare beneficiaries and limit copays on insulin for millions of Americans to $35 each month; and limit annual price increases to stop price gouging by drug corporations. “If Congress lets the pharmaceutical industry overcharge Americans and dictate astronomical prices for brand-name drugs in our country, then patients, workers, employers, and taxpayers will continue to shoulder the burden of prices that are nearly three times what people in other comparable nations pay,” the letter reads. “These reforms are long overdue. We urge swift action. Now is the time to get the job done.” Read the full letter and list of signers here and below. |
| February 7, 2022 Dear Senator, On behalf of more than 90 organizations representing patients, consumers, seniors, unions, small businesses, large employers, physicians, and disease advocacy groups, we urge the Senate to immediately advance a reconciliation package that includes the reforms to lower prescription drug prices agreed to in the Build Back Better Act. Rising prices are a top concern for American families and employers. Even as we approach the third year of a pandemic, drug companies began the new year by hiking the prices of almost 750 life-saving and life-sustaining medications, undeterred by the financial hardship and health challenges facing Americans today. If Congress lets the pharmaceutical industry overcharge Americans and dictate astronomical prices for brand-name drugs in our country, then patients, workers, employers, and taxpayers will continue to shoulder the burden of prices that are nearly three times what people in other comparable nations pay. Congress has repeatedly promised to address this problem, and the American people need the help now more than ever. Right now, you have a time-limited opportunity to deliver relief to millions of Americans by permitting Medicare to negotiate for lower drug prices, capping copays and out-of-pocket costs for beneficiaries, and limiting annual price increases on life-saving and life-sustaining drugs to the rate of inflation for all Americans. More than 80 percent of Americans support these reforms — Democrats, Republicans, and independents alike. They are the most popular element of the Build Back Better Act. In the face of slim margins in the Senate, the current path to pass all of these critical reforms is through reconciliation. These reforms already have the support of all 50 Senate Democrats. Congress can pass these drug pricing reforms in short order and move the nation in a new direction, providing savings to patients, taxpayers, workers, employers, and the federal and state governments. Enacting the drug price reforms agreed upon in the Build Back Better package will mark a truly historic shift in U.S. drug pricing policy. Not only will it break the pharmaceutical industry’s unilateral power to dictate prices to the American people, it will save lives, improve health, fight inflation, and put more money back into the pockets of American seniors, workers, and businesses. These reforms are long overdue. We urge swift action. Now is the time to get the job done. Signed: AARP AFL-CIO AFSCME Alliance for Retired Americans American Academy of Neurology American Federation of Teachers American Medical Student Association Americans for Democratic Action, Southern California Be a Hero Blue Shield of California Centennial State Prosperity Center for American Progress Center for Medicare Advocacy Center for Popular Democracy Citizen Action of Wisconsin Colorado Consumer Health Initiative Communications Workers of America (CWA) Community Catalyst Consumer Action COVID Survivors for Change Doctors for America Economic Alliance for Michigan Families USA Florida #insulin4all Florida Alliance for Healthcare Value Generation Patient Greater Philadelphia Business Coalition on Health Health Access California Health Action New Mexico Health Care For All Massachusetts Health Care Voices Health Care Voter Honest Arizona Houston Business Coalition on Health Indivisible Initiative for Medicines, Access, & Knowledge (I-MAK) Insulin For Life International Association of Machinists and Aerospace Workers International Brotherhood of Teamsters International Union, United Automobile, Aerospace & Agricultural Implement Workers of America (UAW) Jewish Alliance for Law and Social Action Justice in Aging Kentuckiana Health Collaborative Knowledge Ecology International (KEI) KS Business Group on Health Lehigh Valley Business Coalition on Healthcare (LVBCH) Little Lobbyists Lower Drug Prices Now (LDPN) Main Street Alliance Maine Consumers for Affordable Health Care Maryland Citizens’ Health Initiative Massachusetts #insulin4all Chapter Medicare Rights Center Metro New York Health Care for All Midwest Business Group on Health MomsRising Montana Association of Health Care Purchasers National Alliance of Healthcare Purchaser Coalitions National Committee to Preserve Social Security and Medicare National Education Association National Multiple Sclerosis Society Nevada Business Group on Health New Jersey Citizen Action North Carolina Business Group on Health Our Revolution Patients For Affordable Drugs Now Pennsylvania Health Access Network People’s Action Pittsburgh Business Group on Health PrEP4All Prescription Justice Progressive Democrats of America Protect Our Care Public Citizen Purchaser Business Group on Health Rhode Island Business Group on Health Salud y Fármacos SEIU Silicon Valley Employers Forum Social Security Works T1International TakeAction Minnesota Tennessee Health Care Campaign The Alliance (WI, IL and IA) UnidosUS Action Fund UNITE HERE United Mine Workers of America United States of Care Washington Health Alliance WellOK – The Northeastern Oklahoma Business Coalition West Health Institute Cc: The Honorable Joseph R. Biden, Jr., President of the United States The Honorable Nancy Pelosi, Speaker of the U.S. House of Representatives |
| ### |